-
Notifications
You must be signed in to change notification settings - Fork 49
P2D_model
LIONSIMBA provides a simulation environment for Li-ion cells. The specific modelling approach lies on the so-called electrochemical pseudo two-dimensional (P2D) model which is represented by means of Partial Differential and Algebraic Equations (PDAEs).
In this section a brief description of numerical approach used to implement the P2D model is provided.
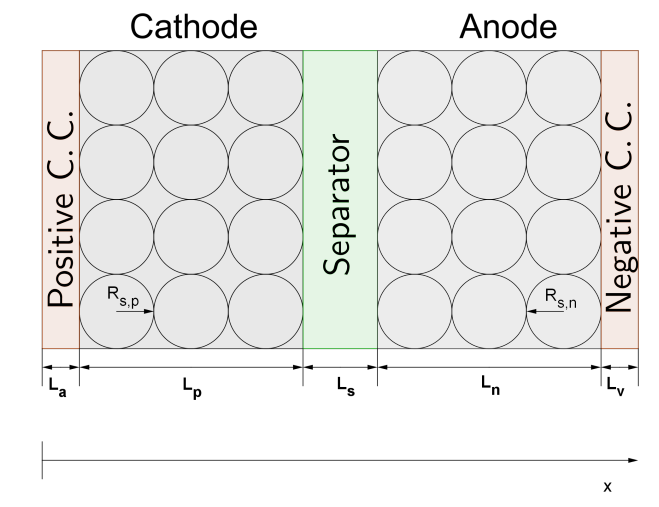
As shown in the figure below, the Li-ion cell is composed of five main sections: the positive current collector (a), the cathode (p), the separator (s), the anode (n), and the negative current collector (v). The index
is used to indicate the different sections. The thickness of each battery section is defined by
and
represents the overall battery thickness. The electrodes and the porous separator of the Li-ion cell are placed in contact with an electrolyte solution, facilitating the flow of ions during charging and discharging processes. During a charging process, the ions deintercalate from the positive electrode and, passing through the porous media of the separator, intercalate into the negative electrode. The inverse process occurs when discharging the cell.
 The diffusion of ions within the electrodes are modeled by
The diffusion of ions within the electrodes are modeled by
where
represents the time,
is the one-dimensional spatial variable,
and
are the surface and average concentration of solid particles respectively, the function
represents the ionic flux, and
and
account for the particle radius and effective diffusion coefficients of the solid phases. The bulk State of Charge (SOC) of the anode is defined as
where represents the maximum concentration of Li-ions in the negative electrode. The flow of ions inside the electrolyte solution is modeled by a diffusion equation,
where represents the electrolyte concentration of ions,
defines the transference number,
is the particle surface area to volume ratio,
accounts for the effective diffusion coefficients in the electrolyte, and
represents the material porosity. According to Ohm's law, the conservation of charge in the electrodes can be defined as
where is the solid potential,
is the electrodes effective conductivity, and
is the Faraday's constant. The potential of the Li-ion cell is obtained as
Similarly, a modified Ohm's law is used to represent the charge conservation within the electrolyte:
where is the electrolyte potential,
represents the temperature,
defines the universal gas constant, and
is the effective conductivity of the liquid phase. The temperature dynamics are modeled by an energy balance,
where is the material density,
is the specific heat,
is the heat diffusion coefficient, and the terms
,
, and
account for ohmic, reversible, and reaction heat sources as shown in Kumaresan et.al, 2008.
The above equations are coupled by means of the ionic flux, which is defined by the Butler-Volmer equation
where the exchange current density is given by
The overpotential at the anode side is defined as
while at the cathode side as
where the terms and
represent the cathode and anode OCV respectively, and
represents the effective kinetic reaction rate. The intercalation flux
is zero inside the separator.
A more detailed explanation of the model, parameters and its numerical implementation are give in the related LIONSIMBA article.